BLOG
Special Report: 12 Notes About TMS
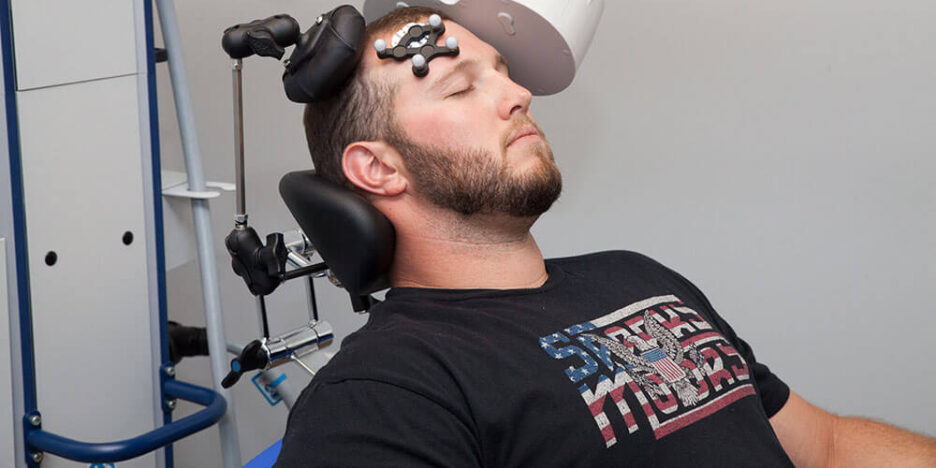
What is transcranial magnetic stimulation? How does it work? Why is it so effective at treating major depressive and obsessive-compulsive disorders? And what other serious mental health conditions might benefit from this promising therapeutic modality?
1. The Electric Brain: As psychiatrists, we know our medications and psychotherapy inside and out, but there is a third approach to treatment that is available to us but underutilized: brain stimulation. This emerging subspecialty, which is in the process of gaining accreditation from the United Council for Neurologic Subspecialties (Siddiqi, et al., 2025), leverages highly effective tools that speak the language of the brain. Electricity, or current flow, is the medium of neuronal signal propagation. It is how the electrochemical organ that we call the brain transmits messages to itself and the rest of the body. Where chemicals merely modulate the efficiency of transmitted messages, electricity mediates this transmission.
Brain stimulation tools for psychiatric disorders currently include two surgical options—deep brain stimulation (DBS) for obsessive-compulsive disorder (OCD) and vagus nerve stimulation (VNS) for major depressive disorder (MDD)—and two non-invasive options: electroconvulsive therapy (ECT) for MDD, bipolar disorder, psychotic disorders, and catatonia, and transcranial magnetic stimulation (TMS) for MDD and OCD, with many other disorders being investigated.
TMS is the only one of these that does not use electricity directly but rather—utilizing Faraday’s law of induction—delivers magnetic waves that pass easily through scalp and skull to induce focal depolarization of underlying neurons (Figure 1). In turn, these neurons can activate downstream neurons through synaptic connections in a circuit. Done repeatedly, these TMS pulses, TMS can modulate brain networks and behavior (Figure 2). This report aims to highlight how TMS is becoming maximally effective through leveraging its rate-limiting, underlying mechanism of action.
2. TMS Is Effective: In real-world practice, 50 to 60% of depressed patients who undergo TMS have a meaningful improvement of more than 50%, and about half of these experience full remission. Because these patients have typically been failed by four or more medications, this represents a substantially higher likelihood of response in contrast with additional medication attempts, as indicated by the STAR-D trial (Rush, et al., 2006). In fact, even after only two medication failures, TMS outperformed medication change or augmentation in two recent large randomized controlled trials (Dalhuisen, et al., 2024; Papakostas, et al., 2024). Notwithstanding the current benefits of TMS, it may be surprising to note that conventional daily standard-of-care TMS protocols have barely changed the original parameters (e.g., intensity, frequency, pulse number, session number, etc., reviewed in Caulfield & Brown, 2022), and improvements (or lack thereof) in effectiveness have followed suit, with one exception.
3. More Sessions: In contrast with medication studies that define the maximum recommended dose based on either no additional benefit or increasing side effects, the dose-response curve of TMS has yet to be clearly established. The upper limits of this curve were tested with the protocol known as Stanford accelerated intelligent neuromodulation therapy, or SAINT/SNT. SAINT radically condensed the dose equivalent of 2.5 months of TMS into five days by giving 10 sessions per day (50 days’ or 10 weeks’ worth) (Cole, et al., 2020). One could even argue that tripling the number of intermittent theta burst stimulation (iTBS) pulses per session (from 600 to 1,800) made this protocol equivalent to 7.5 months’ worth, although there is no clinical data to suggest that 1,800 pulses is more effective than 600. This innovative protocol led to an FDA clearance after a sham-controlled RCT produced nearly 70% response and 50% remission rates at the four-week primary outcome timepoint from 14 subjects receiving active TMS (Cole, et al., 2022).
Interestingly, these results are in line with expected response and remission rates after 50 sessions of daily TMS as well (Hutton, et al., 2023). That is, many patients who have not responded to a standard 36-session course (dictated by insurance coverage in the United States) will become responders with continued treatment in observational studies (Hutton, et al., 2023). Although the end point might be the same, the advantage of the one-week SAINT protocol is sorely needed rapid results.
4. Like an Automobile: If TMS is like an automobile and clinical effectiveness were compared to speed, more TMS sessions would be like “flooring it” (pushing the gas pedal all the way down to the floor). To carry this analogy further, the speed achieved by “flooring it” in an early Model T Ford can only produce a fraction of the speed of a common modern car. What accounts for the difference between them? Among the many optimized parts or “mechanisms”, it is the engine—the driving, rate-limiting mechanism—that underlies automobile performance. Like cars and TMS and just about everything else, if we want to make it perform better, we must first understand how it works.
5. How Does TMS Work? TMS may still be in its relatively early days, but like the automobile in its early days, it’s already a game changer. Following the progression of the auto, TMS appears to be entering a new era, with some recent discoveries that promise big steps forward. To understand these discoveries, we must first understand something of what TMS treatment may be doing to the brain. From the top down, behavior corresponds to relevant brain network function. Networks are comprised of circuits, which are made up of neurons and their connections—synapses (Figure 2). When a synaptic connection changes in strength (referring to how efficiently presynaptic neurotransmitter release causes post-synaptic signal propagation, leading to initiation of the next action potential), synaptic plasticity is said to occur (reviewed in Brown, Higgins, et al., 2021).
6. A Look at Synaptic Plasticity: Synaptic plasticity can be experimentally induced but is also the cellular basis of learning and memory—including adaptive and maladaptive behavioral and thought patterns. Synaptic plasticity includes many different processes, but the classic and best-studied form refers to an increased number of AMPA (α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid) receptors in glutamatergic synapses. More AMPA receptors (AMPARs) means more signal detection, which means greater efficiency. We call this long-term potentiation (LTP). Such synaptic connections within a circuit might constitute something like a healthy thought of gratitude, but could also represent a ruminating worry or a traumatic memory. Synaptic plasticity also refers to a decrease in AMPA receptors and signal sensitivity, called long-term depression (LTD). Not surprisingly, these signals are tightly controlled. Otherwise, learning and memory would be indiscriminate.
Central to both LTP and LTD is the NMDA (n-methyl-d-aspartate) receptor, which has a co-incident detection system that reduces the possibility of inadvertent signals being incorporated. The NMDA receptor (NMDAR) requires glutamate binding and ejection of Mg2+ from its pore via depolarization, which is primarily accomplished by sodium admitted by glutamatergic AMPARs. This means that a subthreshold “weak” signal might be enough to propagate a signal but is not necessarily sufficient to open the calcium-admitting NMDAR. When calcium is admitted entry, the effects depend on the quantity and acuity of calcium in the dentritic spine. Small, chronic amounts of calcium in the post-synaptic dendritic spine bind with calmodulin and activate phosphatases (e.g., calcineurin), which remove phosphate from the AMPAR tail, signaling the cellular machinery to remove it from the synapse. Large, acute influx of calcium bound to calmodulin preferentially activates kinases (via surpassing the threshold to induce non-linear self-recruitment), which attaches a phosphate group to signal machinery to insert an AMPAR into the synapse.
Synaptic plasticity also produces structural changes to dendritic spines—in both size and number. Gene transcription and protein translation are necessary to sustain long-term changes, but these are downstream effects typically activated by synaptic events (Brown, Higgins, et al., 2021). Therefore, at least in theory, synaptic plasticity is the most likely “engine” of TMS.
7. Does TMS Work Through Synaptic Plasticity? Several lines of evidence suggest that the idea of synaptic plasticity as the underlying (or rate-limiting) mechanism of TMS may be more than just theoretical. The most specific evidence comes from mice, in which both 10-Hz repetitive (r)TMS and iTBS protocols have produced an increase in AMPAR levels in the synapse along with other LTP-associated changes (Lu, et al., 2025; Vlachos, et al., 2012). Assays of cortical excitability before and after TMS and in combination with receptor manipulation can yield indirect insights into the rate-limiting mechanism of TMS. In humans, iTBS-induced enhancement (i.e., LTP-like) and inhibition (i.e., LTD-like) were both blocked with an NMDAR antagonist (Huang, 2007). (Because LTP and LTD are technically experimental protocols, and because we don’t directly measure the neuronal markers of LTP and LTD, we use the terms “LTP-like” and “LTD-like.”) NMDAR dependence is a common finding in LTP experiments. However, while many proteins are necessary for synaptic plasticity, only a few are both necessary and sufficient—which are terms used in synaptic plasticity literature to describe the relevance of a given protein in LTP or LTD. An analogy for this is that many educational steps are necessary to become a psychiatrist, but only completing a psychiatry residency is both necessary and sufficient; or, keeping with the car analogy, a wheel on the car is necessary but not sufficient for it to go faster.
8. DCS Is Sufficient: The sufficiency of NMDAR agonism to enhance TMS-induced potentiation was tested with the longtime antibiotic D-cycloserine (DCS)—an NMDAR agonist at lower doses. Indeed, 100mg DCS given two hours before TMS was sufficient to enhance TMS-induced plasticity in the motor cortex along with other associated LTP-like changes (Figure 4) (Brown, et al., 2020; Brown, Yuan, et al., 2021; Kweon, et al., 2022). But does enhanced plasticity translate to clinical benefits? A placebo-controlled RCT with 50 depressed subjects found that DCS plus iTBS was again sufficient to improve clinical response compared with active standard-of-care iTBS, with a large effect size of 0.99 while more than doubling response and remission rates (J. Cole, et al., 2022). In a four-week open-label course, DCS plus iTBS generated remission from depression in nine of 12 subjects with MDD (in only 20 sessions), including all of those who had DCS serum levels above 7ug/mL (DeMayo, et al., 2025). These unprecedented improvements over standard-of-care TMS were not limited to depression, as the same group of researchers found similar improvements in OCD (McGirr, et al., 2025), suggesting a potentially transdiagnostic TMS mechanism (Brown & Philip, 2025).
9. Can Any Psychotropic Augment TMS Effects? Might these DCS results simply be an additive effect of adding any psychotropic medication? The relevant negative control was not included in these TMS-plus-DCS trials, and there are surprisingly few trials among many observational reports (profitably reviewed by Kochanowski, et al., 2024), but one trial testing venlafaxine found no additional benefits to TMS, although the low-frequency protocol used is not conventional (Brunelin, et al., 2014). A low signal-to-noise ratio from observational studies does not clearly identify other drug candidates, though a systematic review on pharmacologic augmentation with physiology outcomes identifies a few modest signals from dopaminergic and noradrenergic agonists (Sohn, et al., 2024).
10. Ketamine: Another NMDAR agent, ketamine, has received much attention (but little data) for this possibility, especially given ketamine’s known rapid and effective benefits in depression. Many case reports and case series have shown that patients get better, but this could be from either TMS or ketamine alone (Debowska, et al., 2023). There has only been one controlled trial that gave TMS plus ketamine or TMS alone (open-label), and it found no difference between these groups, which suggests that ketamine added no benefit.
11. Single-Day TMS: If both accelerated TMS protocols have revealed that we can push the “gas pedal” much further, and mechanism-guided augmentation with DCS has given first insights into harnessing the engine of TMS, could combining these be the next wave of TMS innovation? This intriguing possibility is under investigation by several groups testing the potential for an entire course of TMS to be given in a single day in combination with DCS. The only published report to date is a case series of five patients with either MDD or OCD who received 20 sessions of iTBS in one day paired with DCS along with lisdexamfetamine—all of whom achieved remission that lasted at least three months (Vaughn, et al., 2025). More data to come will inform whether this could become a recommended treatment option.
12. Future Directions: DCS enhancement of TMS for depression and OCD is likely just the beginning of mechanism-guided improvements for this promising treatment tool. With trials underway and expanding evidence, it is expected that TMS will eventually gain FDA clearance for many different disorders—and even specific symptoms. Different TMS protocols will likely be used to achieve different outcomes. The parameter space of TMS is vast and the opportunities endless (Caulfield & Brown, 2022), and the addition of adjuncts will likely necessitate greater precision in parameter selection to produce the desired results. ■
Visit Our Blog
